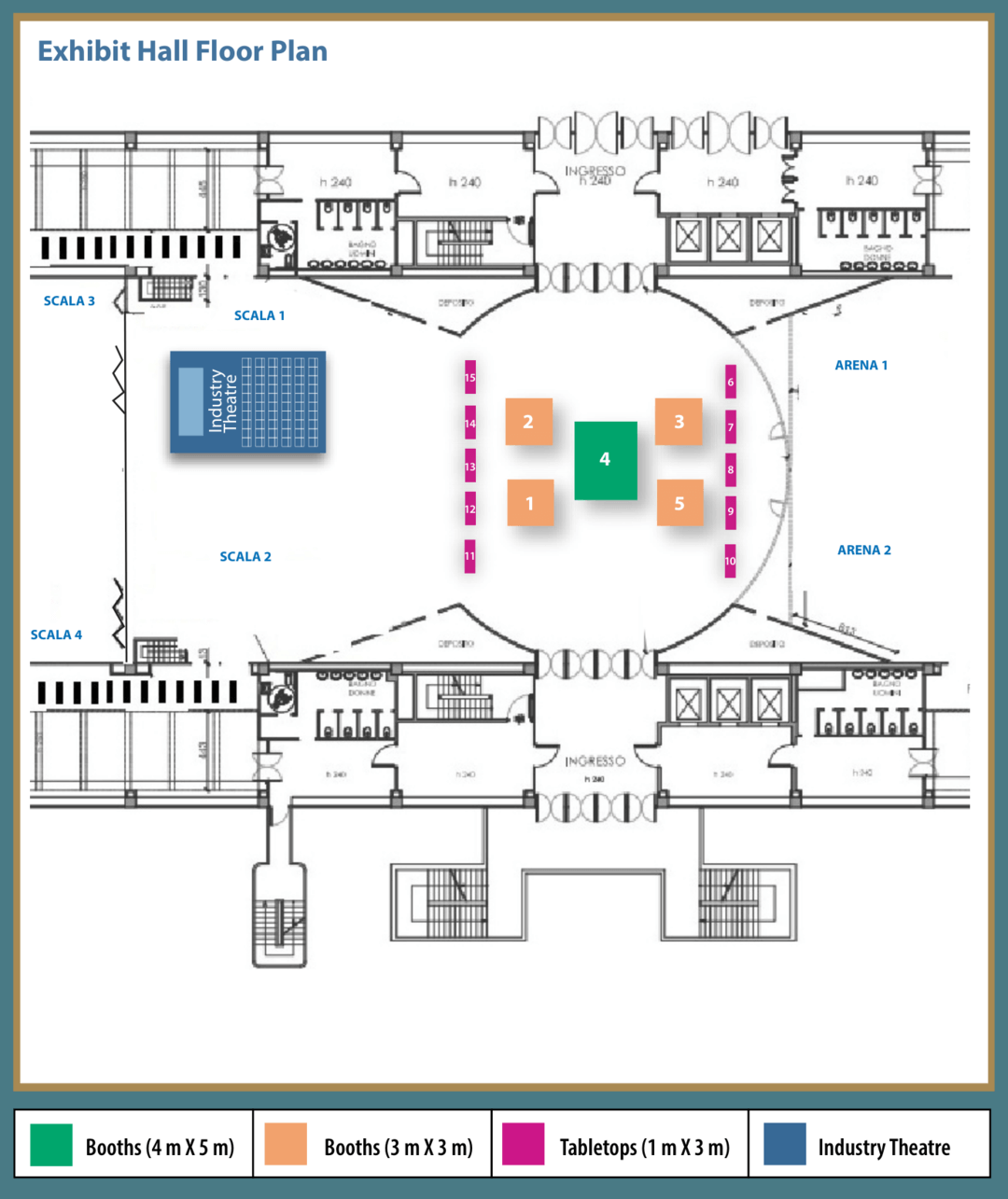
Exhibition and Sponsorship
The International Association of Parkinsonism and Related Disorders (IAPRD) welcomes you to become a supporter of the IAPRD 2026 Congress.
Commercial support is an excellent way for your organization to engage with your audience of clinicians, scientists, allied healthcare providers, residents/fellows and medical students.
The congress features a comprehensive program in movement disorders led by renowned faculty from across the globe with the focus theme “Enhancing Patient Care Through Education, Technology, and Therapeutics.”
The Congress provides opportunities that can enhance your visibility, gain a more powerful presence and increase your impact with IAPRD attendees/members.
Sponsorship Levels
Platinum, Gold, Silver, Bronze (See prospectus for details)
Other Support Opportunities Include:
- Breakfast Session
- Lunchtime Session
- Dinner Session
- Industry Theatre
- Exhibits Booths
- Advertisements
- Branding
- Other Sponsorship Opportunities
Official Italian Agency for AIFA Procedure
Any Italian pharmaceutical company supporting or participating in a congress held in Italy or abroad is subject to authorization by AIFA (the Italian Drug Agency), according to an Italian Government Decree (Decreto Legislativo 219/06 – art. 124). The request for authorization must be submitted at least 60 days before the event’s start date.
Non-Italian pharmaceutical companies are advised to contact the organizing secretariat or the Official Italian Agency appointed for the AIFA submission by July 1, 2026.
The appointed agency to collect all applications from pharmaceutical companies and file them with AIFA is:
AIM Group International – AIM Education S.r.l.
Viale Enrico Forlanini, 23 20134 Milan, Italy
Tel. +39 02 56601.1
e-mail: aifa@aimgroup.eu / c.ghidoli@aimgroup.eu
Key Deadlines and Required Documents
If a company requires AIFA authorization but is not registered with AIFA and does not have an Italian affiliate, it must register on the AIFA website and begin the process by July 01, 2026. This is a very long process, taking approximately 120 days.
Timeline
May 15, 2026 (180 days prior): First information about the AIFA procedure should be sent to all sponsoring companies. We recommend informing companies via email, through the “Sponsor” section of the congress website (including our contact information), and by sending a first email of information about AIFA process (we can provide a draft).
- June 14, 2026 (150 days prior): First list of pharma sponsoring companies is due to AIM.
- July 1, 2026: Deadline for pharma companies without an AIFA SIS Code to start the registration process with AIM.
- July 20, 2026 (100 days prior): Deadline for all AIFA procedure documents to be submitted to AIM.
Documents